
Two Powerful Technologies to Effectively Manage Biofilm Within the Dressing1,2
Biofilm is more likely to develop if exudate management is poor. The unique combination of HydrofiberTM technology and MORE THAN SILVERTM technology contributes to the removal of exudate, debris, and bacteria from the wound, and locking them within the dressing. This aids in optimizing the wound environment to promote healing.1,4,5


BIOFILM IS AN ENEMY WORTH TARGETING
Biofilm is present in 78% of Hard-to-heal wounds6

Biofilm is everywhere
In healthcare, biofilm accounts for more than 80% of all microbial infections.7 In nature, 99% of bacteria exist in biofilm.8
Difficult to eradicate
Biofilm is difficult to completely remove, even with debridement. It reforms quickly9 and is a precurser to infection.10 It is tolerant to antiseptics and antibiotics and able to evade the body's immune response.10,11
Delays wound healing12,13
Biofilm creates a sustained but ineffective inflammatory response.14 It also impairs granulation tissue formation and epithelialisation.14
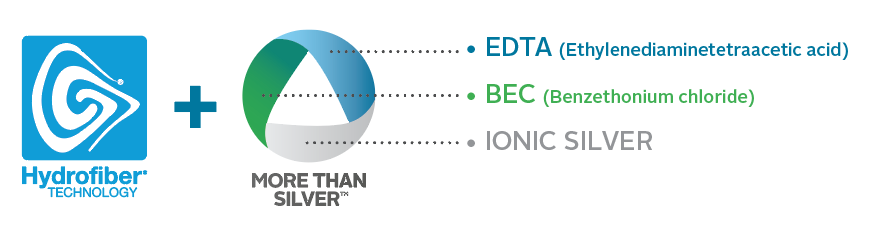
Specifically developed to manage biofilm within the dressing, MORE THAN SILVERTM technology is a unique formulation that contains three components.1
They Synergistically work together to enhance the antimicrobial efficacy of ionic silver within the dressing resulting in:
Faster reduction of bacteria1
Broad spectrum efficacy1, 15, 16
Sustained antimicrobial activity1
DONT WAIT:
Target your enemy with
AQUACEL® Ag Advantage

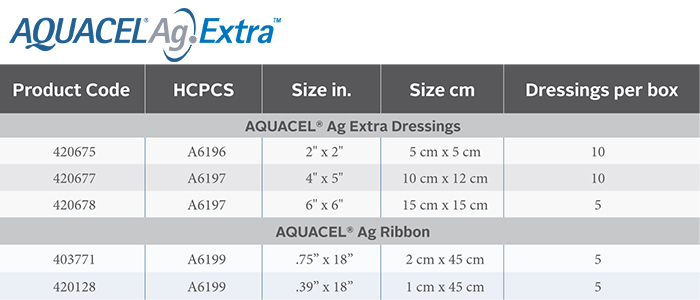
Get your AQUACEL® supplies from Byram by Filling out the form or call 1-800-364-6057
dressing. Wound Medicine. 2016; 14: 6–11.
2. WHRI4796 MA271 – data on ¬file.
3. Hurlow, Bowler. Potential implications of bio¬film in chronic wounds: a case series. J Wound Care 2012; 21: 109-119.
4. Parsons D, Meredith K, Rowlands VJ, Short D, Metcalf DG, Bowler PG. Enhanced Performance and Mode of Action of a Novel Antibio¬film Hydro¬fiber® Wound Dressing. Biomed Res Int. 2016;2016:7616471.
5. Metcalf DG, Parsons D, Bowler PG. Clinical safety and effectiveness evaluation of a new antimicrobial wound dressing designed to manage exudate,
infection and bio¬film. Int Wound J 2017; 14: 203-213.
6. Malone M et al. 2017. The prevalence of bio¬film in chronic wounds: a systematic review and meta-analysis of published data. JWC; 20-25.
7. National Institutes of Health, 2002. Research on microbial bio¬films. https://grants.nih.gov/grants/guide/pa- les/PA-03-047.html. Accessed October
2017.
8. Donlan RM, Costerton JW. Bio¬films: survival mechanisms of clinically relevant microorganisms. Clin Micro Rev. 2002; 15:167-193.
9. Wolcott RD et al. Bio¬film maturity studies indicate sharp debridement opens a time dependent therapeutic window. J Wound Care. 2010; 19:320-328.
10. Percival SL, Bowler PG, 2004. Bio¬films and their potential role in wound
healing. WOUNDS, 16: 234-240.
11. Bowler. Antibiotic resistance and bio¬film tolerance: a combined threat in the treatment of chronic infections. JWC Vol 27;No 5; 2018.
12. Hurlow, J., Couch, K., Laforet, K., Bolton, L., Metcalf, D. et al. (2015). Clinical Biofilms: A Challenging Frontier in Wound Care. Advances in Wound Care, 4(5), 295-301.
13. Metcalf, Bowler. Bio¬film delays wound healing: a review of the evidence. Burns Trauma 2013; 1: 5-12.
14. Gurjala AN et al. Development of a novel, highly quantitative in vivo model for the study of bio¬film-impaired cutaneous wound healing. Wound Rep Reg
(2011) 19 400-410.
15. Antimicrobial activity and prevention of bio¬film reformation by AQUACEL® Ag+ Extra™ dressing. Scienti¬fic Background Report. WHRI3857 MA236, 2013, Data on ¬ le, ConvaTec Inc.
16. Antimicrobial activity against CA-MRSA and prevention of bio¬film reformation by AQUACEL® Ag+ Extra™ dressing. Scienti¬fic Background Report.
WHRI3875 MA239, 2013, Data on file, ConvaTec Inc. *AQUACEL® Ag Advantage is not a drug. All anti-microbial activity occurs within the dressing. The correlation between in-vitro testing and clinical effectiveness has not been determined. AP-030617-MRL-US

